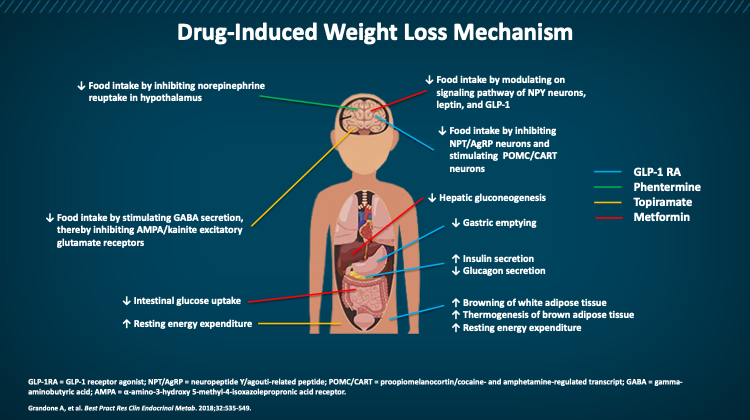
In November 2023, the FDA approved tirzepatide, a single molecule dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist or patients with obesity. Due to its agonistic properties at the GLP-1 receptor and the GIP receptor, it produces additive effects on body weight compared to the GLP- 1 analogues alone.21
Results from the multicenter, randomized, double-blind, placebo-controlled SURMOUNT-1 trial in patients with obesity or overweight plus at least one comorbidity (but not diabetes) demonstrated average weight reductions at 72 weeks of 15.0%, 19.5%, and 20.9% with 5 mg, 10 mg and 15 mg doses of tirzepatide, respectively, compared to a 3.1% weight reduction with placebo. For the prespecified exploratory end point of a reduction in body weight of 25% or more, 15%, 32%, and 36% of participants receiving 5 mg, 10 mg, or 15 mg tirzepatide doses of tirzepatide, respectively, met this target. Weight loss of this magnitude has not been reported in any other trials for antiobesity medications.22
Tirzepatide was also evaluated in the SURPASS-1 and SURPASS-2 trials in patients with obesity and diabetes not adequately controlled with lifestyle changes. In SURPASS-1, patients on tirzepatide demonstrated a weight change from baseline of -7.0 kg, -7.8 kg and -9.5 kg in the 5 mg, 10 mg, and 15 mg groups, respectively, vs -0.7 kg on placebo by the end of week 4, with weight loss continuing until week 40. In addition, 67% to 78% reached a weight loss ≥ 5%, while 31% to 47% reached a weight loss ≥ 10%. Between 13% and 27% reached a weight loss ≥ 15% (13%–27%) compared to 14%, 1% and 0% of participants in the placebo group, respectively.23 Similar results were seen in SURPASS-3.24
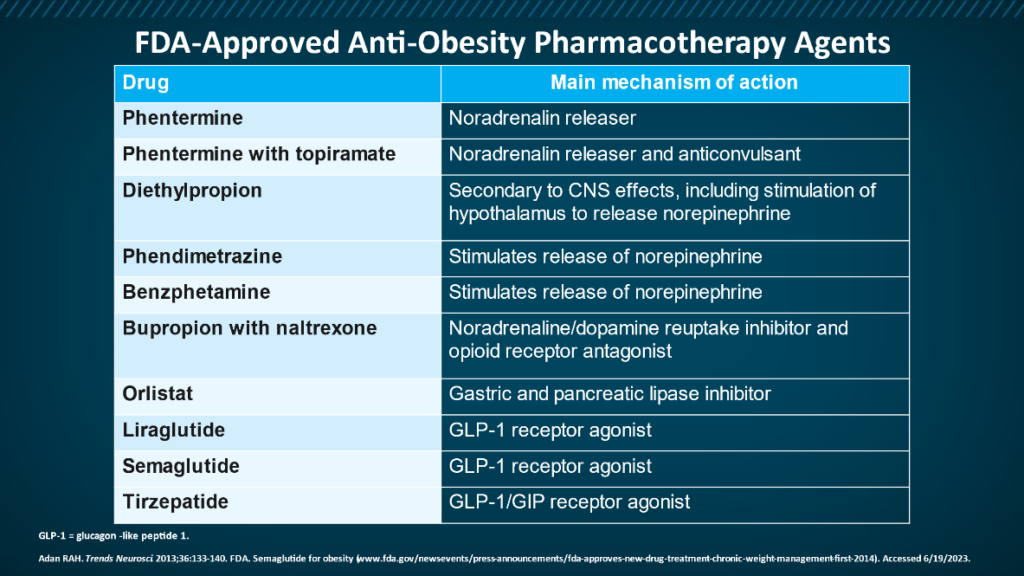
For many people, lifestyle changes alone are not enough to combat obesity. The good news is that today we have several medications available that, together with lifestyle changes, can help you lose weight and keep it off. Most work on the hormones and peptides that help regulate appetite. A couple are injectable, but oral versions are coming. Among the FDA-approved medications for obesity are:
- Orlistat
- Phentermine/topiramate ER
- Naltrexone/bupropion ER
- Liraglutide
- Semaglutide
- Tirzepatide
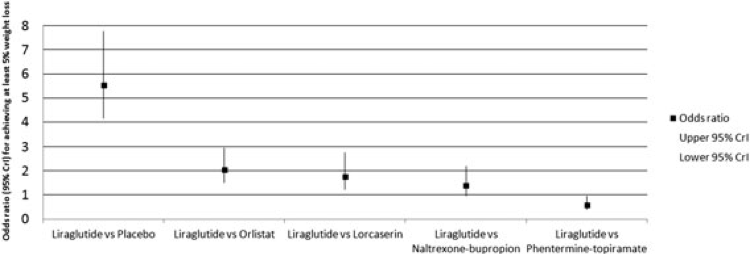
They work in different ways and have different side effects. So talk to your healthcare provider about whether medication is right for you and which medication is best. Always ask about potential side effects and how to manage them.
Tirzepatide was also investigated in the SURMOUNT-3 and SURMOUNT-4 phase 3 studies. SURMOUNT-3 participants (n=806) completed a 12-week intensive lifestyle intervention. After 12 weeks, 579 participants achieved at least a 5% body weight reduction and were randomized to tirzepatide or placebo. Those taking tirzepatide lost an additional 21.2% mean body weight vs those on placebo, who experienced a mean weight regain of 3.3%.
SURMOUNT-4 evaluated the efficacy and safety of tirzepatide compared to placebo for 52 weeks after a 36-week, open-label tirzepatide lead-in period in which all participants (n=783) took tirzepatide, achieving a 21.1% mean weight loss. Then 670 participants were randomized to tirzepatide or placebo for 52 weeks. Those taking tirzepatide lost an additional 18.4% of their body weight on average from randomization vs a mean weight regain of 2.5% over 72 weeks in the placebo group.