Current Strategies in Managing Obesity
Current treatment recommendations call for weight loss interventions in those with a BMI > 25 kg/m2 and one or more cardiovascular or obesity-related risk factors (including elevated waist circumference) or complications; and in those with BMI ≥ 30 kg/m2 regardless of the presence of cardiometabolic risk factors. For individuals of Asian descent, a BMI ≥ 23 kg/m2 should trigger interventions.8, 9
Determining which weight loss interventions to offer depends on the individual’s BMI as well as comorbidities, as shown in Table 2.
Lifestyle Interventions
There are 3 components to lifestyle interventions: reduced caloric intake; increased activity; and behavioral interventions.
Reducing caloric intake involves setting calorie limits, cutting calories, or restricting certain food types such as dietary fat. There is no consensus on which dietary approach is best; that should be determined in discussion with the patient and based on their health status and preferences.
Individuals with obesity should be counselled to accumulate at least 150 minutes a week of moderate aerobic exercise, such as walking, and to engage in resistance training to preserve lean mass. Once the goal weight is achieved, they should increase their moderate aerobic exercise to 200 to 300 minutes a week for maintenance.8,9
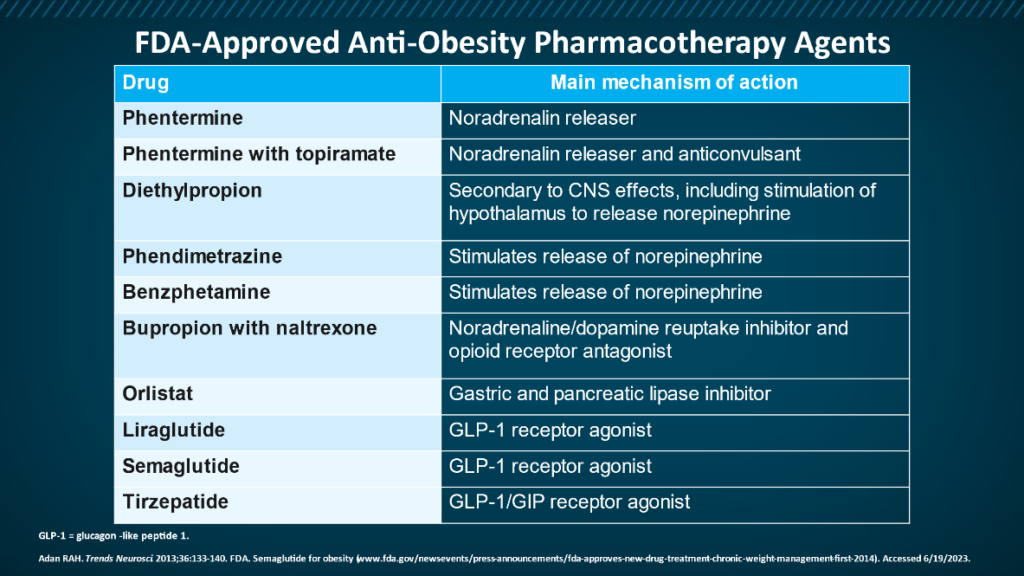
Weight Loss Medications
Other medications used off label to treat overweight/obesity include topiramate, zonisamide, bupropion, metformin, pramlintide, and the SGLT2 Inhibitors canagliflozin and dapagliflozin.14
Liraglutide and semaglutide, originally developed for people with Type 2 diabetes mellitus (T2DM), are approved for chronic weight management in people with obesity (or overweight and ≥ 1 weight-related comorbidities. 15, 16 GLP-1, endogenously produced in the L-cells of the distal ileum and colon, modulates appetite leading to decreased food intake and thus body weight.
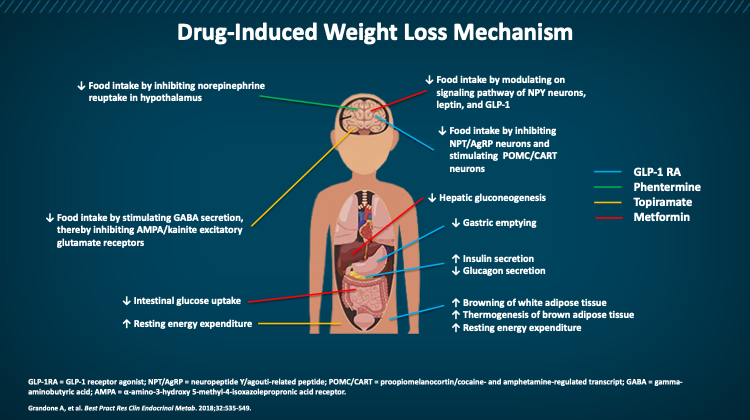
Centrally, GLP-1 promotes satiety through the activation of GLP-1 receptors in the hypothalamus and brainstem, which causes a reduction in food intake. These agents have been demonstrated to modulate appetite, taste preference, gut hormones, and regional body fat stores in adults with obesity without reduction in lean body mass.17Figure 4 depicts the mechanism of action of the GLP-1s and other weight loss medications.
Semaglutide
Once weekly semaglutide was evaluated in the STEP-1, phase 3, double-blind placebo-controlled study, which enrolled 1,961 adults with a BMI of 30 or greater (≥ 27 in persons with ≥ 1 weight-related coexisting condition), who did not have diabetes. They were randomly assigned in a 2:1 ratio, to 68 weeks of treatment with semaglutide (at a dose of 2.4 mg) or placebo, plus lifestyle intervention.19
The mean change in body weight from baseline to week 68 was −14.9% in the semaglutide group compared with −2.4% with placebo, for an estimated treatment difference of −12.4 percentage points (95% confidence interval [CI], −13.4 to −11.5; P < .001). More participants in the semaglutide group than in the placebo group achieved weight reductions of 5% or more (1047 participants [86.4%] vs 182 [31.5%]), 10% or more (838 [69.1%] vs 69 [12.0%]), and 15% or more (612 [50.5%] vs 28 [4.9%]) at week 68 (P < .001 for all three comparisons). The change in body weight from baseline to week 68 was −15.3 kg in the semaglutide group compared with −2.6 kg in the placebo group (estimated treatment difference, −12.7 kg; 95% CI, −13.7 to −11.7). Participants who received semaglutide had a greater improvement in cardiometabolic risk factors and a greater increase in participant-reported physical functioning from baseline than those who received placebo.19
The most common adverse events with semaglutide was transient, mild-to-moderate nausea and diarrhea, leading to a discontinuation rate of 4.5% vs 0.8% in the placebo group.19
Liraglutide
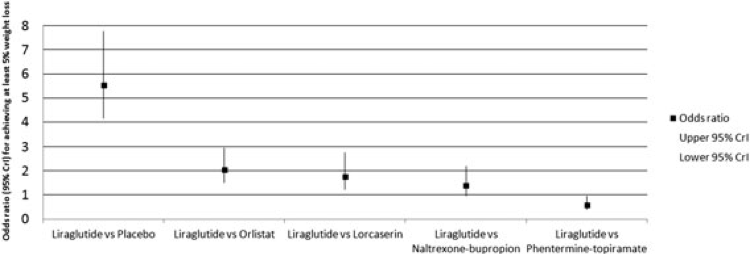
Liraglutide has been evaluated for weight loss in at least 5 randomized, placebo-controlled, Phase 3 clinical trials, including 1 in participants with sleep apnea, 1 in those with pre-diabetes, and 1 in those with diabetes. Participants were primarily women in most of the studies. Figure 5 shows the odds ratios and 95% credible intervals (CrI) for achieving at least 5% weight loss at one year in phase III clinical trials for liraglutide.20
Liraglutide resulted in a 4 to 6 kg weight loss throughout trials, with a significantly higher percentage of participants achieving at least 5% and 10% weight loss compared with placebo. The most common adverse effects were gastrointestinal, which primarily occurred early in the treatment course.20
Tirzepatide
In November 2023, the FDA approved tirzepatide, a single molecule dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist or patients with obesity. Due to its agonistic properties at the GLP-1 receptor and the GIP receptor, it produces additive effects on body weight compared to the GLP- 1 analogues alone.21
Results from the multicenter, randomized, double-blind, placebo-controlled SURMOUNT-1 trial in patients with obesity or overweight plus at least one comorbidity (but not diabetes) demonstrated average weight reductions at 72 weeks of 15.0%, 19.5%, and 20.9% with 5 mg, 10 mg and 15 mg doses of tirzepatide, respectively, compared to a 3.1% weight reduction with placebo. For the prespecified exploratory end point of a reduction in body weight of 25% or more, 15%, 32%, and 36% of participants receiving 5 mg, 10 mg, or 15 mg tirzepatide doses of tirzepatide, respectively, met this target. Weight loss of this magnitude has not been reported in any other trials for antiobesity medications.22
Tirzepatide was also evaluated in the SURPASS-1 and SURPASS-2 trials in patients with obesity and diabetes not adequately controlled with lifestyle changes. In SURPASS-1, patients on tirzepatide demonstrated a weight change from baseline of -7.0 kg, -7.8 kg and -9.5 kg in the 5 mg, 10 mg, and 15 mg groups, respectively, vs -0.7 kg on placebo by the end of week 4, with weight loss continuing until week 40. In addition, 67% to 78% reached a weight loss ≥ 5%, while 31% to 47% reached a weight loss ≥ 10%. Between 13% and 27% reached a weight loss ≥ 15% (13%–27%) compared to 14%, 1% and 0% of participants in the placebo group, respectively.23 Similar results were seen in SURPASS-3.24
For many people, lifestyle changes alone are not enough to combat obesity. The good news is that today we have several medications available that, together with lifestyle changes, can help you lose weight and keep it off. Most work on the hormones and peptides that help regulate appetite. A couple are injectable, but oral versions are coming. Among the FDA-approved medications for obesity are:
- Orlistat
- Phentermine/topiramate ER
- Naltrexone/bupropion ER
- Liraglutide
- Semaglutide
- Tirzepatide
They work in different ways and have different side effects. So talk to your healthcare provider about whether medication is right for you and which medication is best. Always ask about potential side effects and how to manage them.
Tirzepatide was also investigated in the SURMOUNT-3 and SURMOUNT-4 phase 3 studies. SURMOUNT-3 participants (n=806) completed a 12-week intensive lifestyle intervention. After 12 weeks, 579 participants achieved at least a 5% body weight reduction and were randomized to tirzepatide or placebo. Those taking tirzepatide lost an additional 21.2% mean body weight vs those on placebo, who experienced a mean weight regain of 3.3%.
SURMOUNT-4 evaluated the efficacy and safety of tirzepatide compared to placebo for 52 weeks after a 36-week, open-label tirzepatide lead-in period in which all participants (n=783) took tirzepatide, achieving a 21.1% mean weight loss. Then 670 participants were randomized to tirzepatide or placebo for 52 weeks. Those taking tirzepatide lost an additional 18.4% of their body weight on average from randomization vs a mean weight regain of 2.5% over 72 weeks in the placebo group.
Emerging Medical Therapies for Obesity
There are several compounds in clinical trials with different targets. They include:
Amylin, a pancreatic hormone co-secreted with insulin, appears to exert a beneficial weight lowering effect that is centrally mediated through appetite control and modulates energy intake. The amylin-based compounds pramlintide, cagrilintide (with or without semaglutide), and setmelanotide are in later-stage clinical trials.2
Other targets under investigation for weight loss therapies include fibroblast growth factor 21 a circulating hormone induced in the fasting state and involved in the metabolic response to fasting; ghrelin antagonism; growth differentiation factor 15; and melanin-concentrating antagonists.2
Metabolic Bariatric Surgery
Current guidelines recommend metabolic bariatric surgery (MBS) for individuals with BMI ≥ 35 kg/m2, regardless of comorbidities and in patients with T2DM and BMI ≥ 30 kg/m2; and considered for individuals with metabolic disease and a BMI of 30-34.9 kg/m2. In the Asian population, a BMI > 25 kg/m2 suggests clinical obesity, and individuals with BMI > 27.5 kg/m2 should be offered MBS.25
Metabolic bariatric surgery has benefits beyond weight loss, with strong evidence that it reverses T2DM and significantly reduces the risk of major CVD outcomes, including coronary artery disease, myocardial infarction, cerebrovascular accident, heart failure, and CVD mortality.26, 27
The long-term weight loss benefits of MBS are well known. A systematic review with meta-analysis on 33 data sets with at least 10 years follow up, including a single center with 20 years of follow-up, found significant and sustained weight loss after surgery. Eighteen reports showed a weighted mean of 56.7% excess weight loss (EWL) with gastric surgery; 17 showed 45.9% EWL with laparoscopic adjustable gastric banding (LAGB); 9 reports of biliopancreatic bypass with/without duodenal switch showed 74.1% EWL; and 2 reports of sleeve gastrectomy showed 58.3% EWL. At the single center with 20 years followup, the 35 patients followed had weight loss of 30.1 kg, 48.9% EWL and 22.2% total weight loss.28
References
- Centers for Disease Control and Prevention. Adult Obesity Facts. Accessed June 26, 2023, https://www.cdc.gov/obesity/data/adult.html
- Abdel-Malek M, Yang L, Miras AD. Pharmacotherapy for chronic obesity management: a look into the future. Intern Emerg Med. 2023;doi:10.1007/s11739-023-03237-4
- Marx J. Cellular warriors at the battle of the bulge. Science. 2003;299(5608):846-9. doi:10.1126/science.299.5608.846
- Authesserre N, et al. Cell Biology Promotion. Accessed June 27, 2023, www.cellbiol.net/ste/alpobesity2.php
- Berg S. AMA: Use of BMI alone is an imperfect clinical measure. Accessed June 25, 2023, https://www.ama-assn.org/delivering-care/public-health/ama-use-bmi-alone-imperfect-clinical-measure
- Centers for Disease Control and Prevention. Defining adult overweight & obesity. Accessed June 27, 2023, www.cdc.gov/obesity/adult/defining.html
- Stanford FC, Lee M, Hur C. Race, Ethnicity, Sex, and Obesity: Is It Time to Personalize the Scale? Mayo Clin Proc. 2019;94(2):362-363. doi:10.1016/j.mayocp.2018.10.014
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63:2985-3023;10.1016/j.jacc.2013.11.004
- Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203.doi:10.4158/ep161365.Gl
- Apovian CM, Garvey WT, Ryan DH. Challenging obesity: Patient, provider, and expert perspectives on the roles of available and emerging nonsurgical therapies. Obesity (Silver Spring). 2015;23(suppl 2):S1-S26.doi:10.1002/oby.21140
- Idrees Z, Cancarevic I, Huang L. FDA-approved pharmacotherapy for weight loss over the last decade. Cureus. 2022;14:e29262. doi:10.7759/cureus.29262
- Drugs.com. Phendimetrazine Tablets Prescribing Information. Acertis Pharmaceuticals, LLC; 2022. Accessed June 26, 2023, https://www.drugs.com/pro/phendimetrazine-tablets.html
- Drugs.com. Benzphetamine Prescribing Information. Calvin Scott Inc; 2022. Accessed June 26, 2023, https://www.drugs.com/pro/benzphetamine.html
- Stanford FC, Alfaris N, Gomez G, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: A multi-center study. Surg Obes Relat Dis. 2017;13:491-500.
- Saxenda® (liraglutide). Prescribing information. Novo Nordisk Inc; 2023. https://www.novo-pi.com/saxenda.pdf
- Wegovy® (semaglutide). Prescribing information. Plainsboro, NJ: Novo Nordisk Inc; 2022. https://www.novo-pi.com/wegovy.pdf
- Kadouh H, Chedid V, Halawi H, et al. GLP-1 analog modulates appetite, taste preference, gut hormones, and regional body fat stores in adults with obesity. J Clin Endocrinol Metab. 2020;105:1552-1563. doi:10.1210/clinem/dgz140
- Grandone A, Di Sessa A, Umano GR, Toraldo R, Miraglia Del Giudice E. New treatment modalities for obesity. Best Pract Res Clin Endocrinol Metab. 2018;32:535-549. doi:10.1016/j.beem.2018.06.007
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi:10.1056/NEJMoa2032183
- Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: A critical review of the evidence. Obes Sci Pract. 2017;3:3–14. doi:10.1002/osp4.84
- Holst JJ. Incretin–based therapy of metabolic disease. Dan Med J. 2022;70(1):A10220597.
- Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–216. doi:10.1056/NEJMoa2206038
- Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet. 2021;398:143-155. doi:10.1016/s0140-6736(21)01324-6
- Ludvik B, Giorgino F, Jódar E, et al. Once–weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583-598. doi:10.1016/s0140-6736(21)01443-4
- Eisenberg D, Shikora SA, Aarts E, et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for metabolic and bariatric surgery. Surg Obes Relat Dis. 2022;18:1345-1356. doi:10.1016/j.soard.2022.08.013
- Balasubaramaniam V, Pouwels S. Remission of type 2 diabetes mellitus (T2DM) after sleeve gastrectomy (SG), one-anastomosis gastric bypass (OAGB), and Roux-en-Y gastric bypass (RYGB): A systematic review. Medicina (Kaunas). 2023;59:985. doi:10.3390/medicina59050985
- Chandrakumar H, Khatun N, Gupta T, Graham–Hill S, Zhyvotovska A, McFarlane SI. The effects of bariatric surgery on cardiovascular outcomes and cardiovascular mortality: A systematic review and meta-analysis. Cureus. 2023;15:e34723. doi:10.7759/cureus.34723
- O’Brien PE, Hindle A, Brennan L, et al. Long-term outcomes after bariatric surgery: A systematic review and meta–analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29:3-14. doi:10.1007/s11695-018-3525-0
- Alberga AS, Edache IY, Forhan M, Russell-Mayhew S. Weight bias and health care utilization: a scoping review. Prim Health Care Res Dev. 2019;20:e116. doi:10.1017/s1463423619000227
- Alpert JS. “So, doctor, what’s so bad about being fat?” Combating the obesity epidemic in the United States. Am J Med. 2010;123(1):1-2. doi:10.1016/j.amjmed.2009.05.027
- Magkos F, Fraterrigo G, Yoshino J, et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016;23(4):591-601. doi:10.1016/j.cmet.2016.02.005
- Bartels RD, Kelly KM, Rothman AJ. Moving beyond the function of the health behaviour: the effect of message frame on behavioural decision-making. Psychol Health. 2010;25(7):821-38. doi:10.1080/08870440902893708
- Rothman AJ, Salovey P. Shaping perceptions to motivate healthy behavior: the role of message framing. Psychol Bull. 1997;121(1):3-19. doi:10.1037/0033-2909.121.1.3
- Campbell MK, DeVellis BM, Strecher VJ, Ammerman AS, DeVellis RF, Sandler RS. Improving dietary behavior: the effectiveness of tailored messages in primary care settings. Am J Public Health. 1994;84(5):783-7. doi:10.2105/ajph.84.5.783
- Jay M, Gillespie C, Schlair S, Sherman S, Kalet A. Physicians’ use of the 5As in counseling obese patients: is the quality of counseling associated with patients’ motivation and intention to lose weight? BMC Health Serv Res. 2010;10:159. doi:10.1186/1472-6963-10-159
- Sarwer DB, Gasoyan H, Bauerle Bass S, Spitzer JC, Soans R, Rubin DJ. Role of weight bias and patient-physician communication in the underutilization of bariatric surgery. Surg Obes Relat Dis. 2021;17(11):1926-1932. doi:10.1016/j.soard.2021.07.013
- Gallagher C, Corl A, Dietz WH. Weight Can’t Wait: A Guide to Discussing Obesity and Organizing Treatment in the Primary Care Setting. Obesity (Silver Spring). 2021;29(5):821-824. doi:10.1002/oby.23154